*Cover image courtesy of the National Cancer Institute.
National Brain Tumor Society, in conjunction with neuro-oncologists, scientific researchers, and the biopharmaceutical industry, is constantly looking for new and better treatments for patients. However, scientific progress in a laboratory does not necessarily always lead to new treatments for patients. First, those laboratory results need to be evaluated and confirmed in high quality clinical trials to ensure the new therapy is truly safe and effective. All of the treatments patients use today, like Temodar, Avastin, Lomustine, and Optune, are the result of past successful clinical trails.
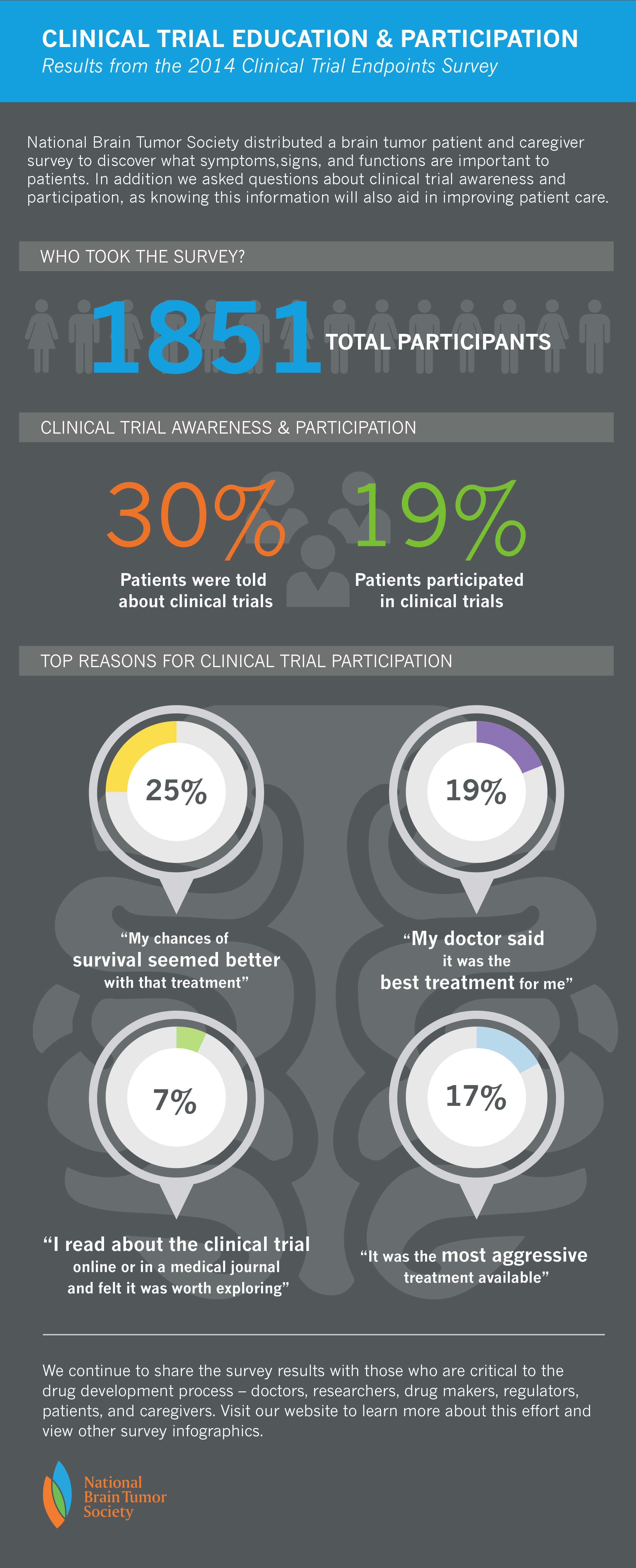
Our latest in a series of infographics developed from the findings of our 2014 Patient and Caregiver Survey highlights what the community told us in regards to their experience with brain tumor clinical trials:
The above information in the infographic represents a summary of all responses for brain tumor patients. However, when broken down by specific tumor type, the data tells different stories:
We encourage patients to ask their doctors about what clinical trials are available for their tumor type. We also encourage doctors to talk to patients about if, and which, clinical trials might be right for them, as well as to help patients find appropriate clinical trials. Lastly, we encourage patients who have participated in clinical trials to speak up and share your experience.
For more information on clinical trails, please see NBTS’s clinical trials, National Cancer Institute’s clinical trials, and CISCRP education. Also, don’t forget to check out the other infographics: the first focused on diagnosis and top priorities for future treatments; the second around caregiver specific responses; and the third on diagnosis and treatments.
Thank you to everyone who took this survey and for helping inform stakeholders – doctors, researchers, drug makers, FDA, and other patient advocacy nonprofits – in brain tumor drug development.
Learn more about the Clinical Trial Endpoints Initiative and please donate so that NBTS can continue to do work like this!